-
TS-1 Molecular Sieve
Sep 18, 2018 | ACS MATERIAL LLCMedium-pore TS-1 is a highly versatile zeolite with MFI structure that has been applied as an efficient catalyst in diverse industrial applications. TS-1 demonstrates unique catalytic performance in oxidation reactions involving H2O2 as an oxidant. TS-1 catalyst is highly effective in the selective oxidation of various organic substrates with hydrogen peroxide, including hydroxylation of aromatics, epoxidation of alkenes, ammoximation of ketones, oxidation of alkanes and alcohols, etc.
Introduction
As an environmentally benign catalyst, the successful synthesis of titanium silicalite molecular sieve (TS-1) has been considered to be a milestone in zeolite catalysis as of the 1980sbecause of its unique catalytic performance in oxidation reaction involving H2O2 as an oxidant.1 TS-1 zeolite has the same skeletal structure (MFI) as ZSM-5. It possesses bi-directional 10-membered ring pore systems with the pore size along (100) as 5.1×5.5 Å and 5.3×5.6 Å along (010) (Figure 1).2 Ti species in TS-1 exist in three different forms, with only the tetrahedral Ti species in the framework being able to express high selectivity while the octahedral Ti species and anatase-TiO2 crystals reduce the utilization of hydrogen peroxide3.
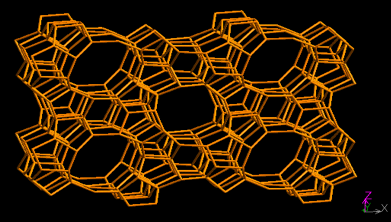
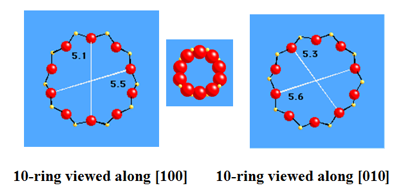
Figure 1. Topology of MFI skeletal structure
Synthesis
Conventional hydrothermal synthesis procedure for the preparation of TS-1 is as follows4: tetraethoxysilane (TEOS) and tetrabutylorthotitanate (TBOT) are first hydrolyzed in an aqueous solution of tetrapropylammonium hydroxide (TPAOH). After removal of the alcohols by evaporation at 343-353 K, a clear gel solution with a molar composition of SiO2/TiO2/TPAOH/H2O=1:0.025:0.15:15 is obtained. The gel is then precrystallized at 443 K for 12 hours. This product, incompletely crystallized but presumably containing at least the primary and secondary building units of the MFI structure, is collected by centrifugation and rinsed several times with distilled water. The white cake is then dispersed in water to obtain a white colloidal solution that is used for crystallization. Fully crystallized TS-1 samples for control experiments are obtained by prolonging the crystallization time to 3 days at 443 K. The calcination of the solid product is performed in air at 823 K for 8 hours.
Many different protocols have been developed for the synthesis of TS-1.5 These include different Si and Ti sources, organic structure directing agents (SDA), and mineralizing agents (see Figure 2).6 From the large number of preparations described in literature, it is possible to find TS-1 materials with different crystal size, morphology, hydrophobic-hydrophilic properties, and titanium distributions along the crystals.7 Two types of TS-1 materials of varying particle sizes ( 0.3-0.5 µm and 20-50 µm) are available for purchase on our ACS Material online store (Figure 3).
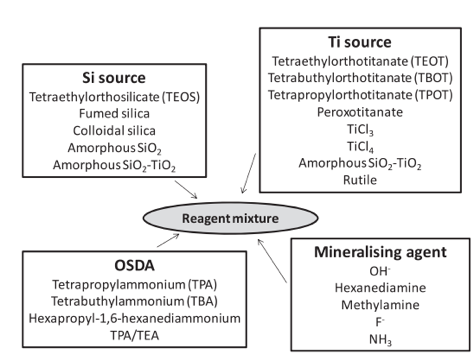
Figure 2. Large number of sources used for TS-1 preparation. Reproduced from ref. 5

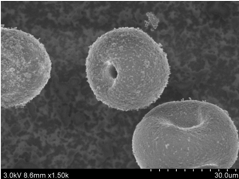
Figure 3. SEM images of ACS Material TS-1 molecular sieves available for purchase.
Applications
TS-1 catalyst is highly effective in the selective oxidation of various organic substrates with hydrogen peroxide such as hydroxylation of aromatics, epoxidation of alkenes, ammoximation of ketones, and oxidation of alkanes and alcohols.
The shape selectivity of TS-1 for oxidation reactions was first shown by Takashi Tatsumi from the Tokyo Institute of Technology Japan when oxidizing different linear, branched and cyclic alkanes with TS-1 using H2O2 as an oxidant (shown in Table 1).8 Hexane showed an oxidation turnover number seventeen times higher than cyclohexane and branched alkanes showed negligible activity. There is also a notable decrease in activity when the length of the linear alkane is above six carbon atoms, in clear relationship with higher diffusion restrictions (see Table 1). Other reactions like the epoxidation of alkenes and the oxidation of unsaturated alcohols have also been tested in order to further study shape-selective oxidation.
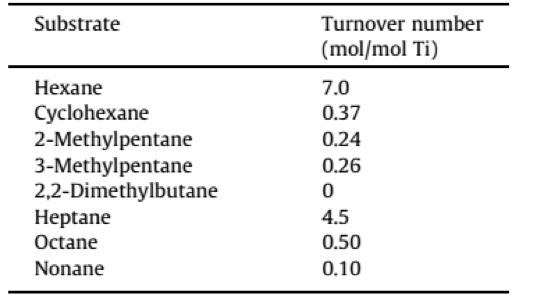
Table 1. Results of the oxidation of different alkanes with TS-1using H2O2 reported by Tatsumi et al. 8
Conclusion
TS-1 molecular sieve is a highly versatile molecular sieve with MFI structure. Many economic and green methods for synthesizing TS-1 are still underway. TS-1 catalyst is highly effective in the selective oxidation of various organic substrates with hydrogen peroxide. Reactions such as the ammoximation of cyclohexanone to the corresponding oxime and synthesis of propylene oxide have been commercialized successfully using TS-1 as catalysts. Investigations about other promising applications of TS-1 are ongoing.
ACS Material Products:
FAQ
Q. What Is a Molecular Sieve?
A. A porous material that has uniformly sized pores that can separate depending on their size. It allows smaller molecules to pass and blocks the larger ones.
Q. Molecular Sieve Binders: What Are They for?
A. Molecular sieve binders play their role in improving the mechanical strength and structural integrity of molecular sieves. They are imperative in shaping the fine sieves powder into extrudates and beads. The binders offer durability, water resistance, and structural integrity.
Q. What Is the Process of Molecular Sieve Adsorption?
A. In molecular sieve adsorption, gas or liquid molecules pass through a material with tiny and uniform pores. Additionally, the pores allow specific molecules to pass through based on their size, while others are blocked. Therefore, it’s ideal for the selective separation or purification of substances.
Q. What Is a Molecular Sieve for CO2 Adsorption?
A. A molecular sieve for CO₂ desorption is a zeolite or activated carbon material with perfectly sized pores that selectively holds CO₂ molecules according to the shape and size.
What Is Molecular Sieve Desiccant?
A. A molecular sieve desiccant is a synthetic crystalline material, occasionally created from zeolite, that has a network of perfectly sized pores. The pores make molecular sieves an effective material for deep drying and moisture control in industrial processes and chemical production.
Q. What is Carbon Molecular Sieve?
A. A carbon molecular sieve CMS is a porous carbon material with a precisely controlled system of micropores that is used for separating gases based on adsorption rate and size. Furthermore, CMS has a narrow distribution, unlike the conventional activated carbon.
Q. How Does a Molecular Sieve Work?
A. Molecular sieves work through a process called adsorption. In this process, molecules are captured in uniform, microscopic pores. As all the pores are the same size, only smaller molecules can enter and be trapped. Therefore, it allows molecular sieves to remove specific compounds, such as CO₂ and water, and other impurities from liquid and gas mixtures.
Q. What Are Molecular Sieve Zeolites?
A. Molecular sieve zeolites are crystalline amorphous minerals that have a uniform three-dimensional pore structure. These highly ordered pores function like tiny molecular filters, allowing for the absorption and separation of molecules based on their shape, size, and polarity.
Q. What Is Molecular Sieve Used for?
A. The primary use of molecular sieves is to function as an agent to remove moisture from air and natural gases. Scientists and researchers use it for drying liquids and gases, as well as separating molecules based on their polarity and size.
References
1. Ratnasamy, P., Kumar. R. “Transition metal-silicate analogs of zeolites”, Catalysis Letter, vol. 22, no. 3, Sep. 1993, pp. 227-237.
2. www.iza-structure.org/databases/
3. Millini, R., Massara, E. P., Perego, G., Bellussi., G.“Framework composition of titanium silicalite-1”, Journal of Catalysis, vol. 137, no.2 , 1992, pp. 497-503.
4. Thangaraj, A., Eapan, M. J., Sivasanker, S., Ratnasamy, R. “Studies on the synthesis of titanium silicalite TS-1”, Zeolites, vol. 12, no. 8, Nov. 1992, pp. 943-950.
5. Tamura, M., Chaikittisilp, W., Yokoi, T., Okubo, T. “Incorporation process of Ti species into the framework of MFI type zeolite”, Microporous and Mesoporous Materials, vol. 112, no. 1-3, Jul. 2008, pp. 202-210.
6. Perego, C., Carati, A., Ingallina, P., Mantegazza, M. A., Bellussi, G. “Production of titanium containing molecular sieves and their application in catalysis”Applied Catalysis A: General, vol. 221, no. 1-2, Nov. 2001, pp. 63-72.
7. Parker, W. O., Millini, R J. “Ti Coordination in Titanium Silicalite-1”, Journal of American Chemical Society, vol. 128, no. 5, Jan. 2006, pp. 1450-1451.
8. Tatsumi, T., Nakamura, M., Negishi, S., Tominaga, H. “Shape-selective oxidation of alkanes with H2O2 catalysed by titanosilicate”, Journal of Chemical Society, Chemical Communication, no. 6, 1990, pp. 476-477.