Single Layer Graphene
Dispersible Single layer Graphene with high surface area (Powder and Dispersion)
Product Detail
CAS No.: 7782-42-5
Single-layer graphene has emerged as a highly capable material that has the ability to replace outdated technologies and benefit countless industries. Incredibly lightweight yet highly durable, graphene is able to conduct a high level of electricity through a minuscule amount of material. Applications of graphene include small electrical circuits and outlets, medical equipment, solar cells, and more. Due to its flexible nature, many industry leaders have begun using graphene as a way to ensure the safety of their various equipment while not risking its efficiency. Single-layer graphene has proven to be an incredibly reliable resource that is both cost-efficient and energy-efficient.
Single layer graphene from ACS Material is available in powder or dispersion forms. The single layer graphene powder is created using a combination of thermal exfoliation reduction and hydrogen reduction. The single layer graphene dispersion is created via our own mechanical stripping and dispersion method. Both products are metal ion free and can be used as conductive agents to improve the high rate charge-discharge capacity of batteries. In addition to batteries, single layer graphene powders and dispersions are used in supercapacitors, lead-acid cells, solar cells, semiconductor chips, graphene films, coatings, as well as in many biomaterials.
Preparation Method
Powder: Thermal exfoliation reduction + Hydrogen reduction
Dispersion: Mechanical stripping and dispersion Method
Characterizations
Table 1 ACS Material Single Layer Graphene Powder
|
Single Layer Graphene Powder/ Monolayer Graphene Powder |
|
|
Flake Diameter (μm) |
0.4-5 |
|
Thickness (nm) |
0.6-1.2 |
|
BET surface area (m2/g): |
400 ~1000 |
|
Electrical resistivity (Ω·cm): |
≤ 0.30 |
|
Density |
~0.01 g/cm3 |
|
Dispersible property: |
Can be re-dispersed in most solvents with the help of sonication |
Table 2 ACS Material Single Layer Graphene Dispersion in DI Water (~~New~~)
|
Single Layer Graphene Dispersion in DI Water |
|
|
Concentration (mg/ml) |
1 |
|
Flake Diameter (μm) |
0.4-5.0 |
|
Thickness (nm) |
0.6-1.2 |
|
DI Water (wt%) |
99.9 |
|
Dispersant (wt%) |
0.1 |
Notes: Please ultrasonicate the single layer graphene dispersion before use.
Dispersible Single Layer Graphene is prepared by completely reducing graphene oxide‚ which is prepared by the modified Hummer’s method. The commonly used prepare method creates a denser graphene which is subject to aggregations. The resulting graphene agglomerates are not soluble or redispersable in water or other polar solvents‚ and this makes further processing difficult. Our Dispersible Graphene avoids this problem and can be redispersed in many solvents.
ACS Material can also provide Fluorinated Graphene. Read More>>

SEM image of ACS Material's Single Layer Graphene
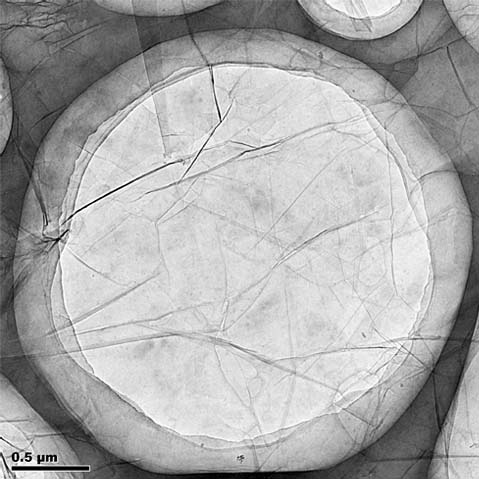
TEM image of ACS Material's Single Layer Graphene (1)
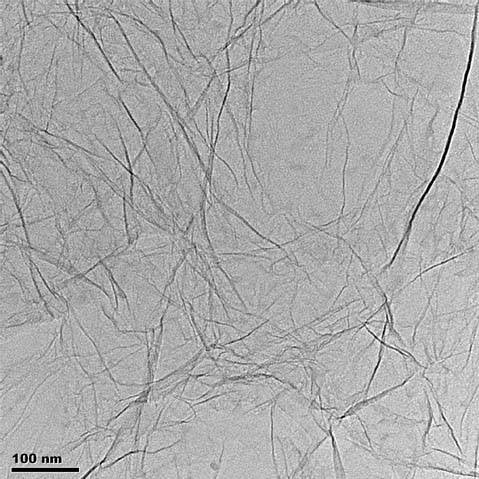
TEM image of ACS Material's Single Layer Graphene (2)
Application Fields
This product is a monolayer graphene-based oily battery slurry with high electrical conductivity made by ultrasonic dispersible Single Layer Graphene. It is metal ion free and can be widely applied in battery slurry as conductive agent to improve the high rate charge-discharge capacity.
1) Lithium ion and nickel-hydrogen battery - as high conductive components in battery slurry
2) Supercapacitors - conductive reagents of the supercapacitor electrodes
3) Catalyst
4) Lead acid cell, Solar energy, Solar Cell
5) Graphene semiconductor chips and semiconductor industry
6) Conductive graphene film
7) Graphene computer memory
8) Biomaterials
9) Transparent conductive coatings
Disclaimer: ACS Material LLC believes that the information on our website is accurate and represents the best and most current information available to us. ACS Material makes no representations or warranties either express or implied, regarding the suitability of the material for any purpose or the accuracy of the information listed here. Accordingly, ACS Material will not be responsible for damages resulting from use of or reliance upon this information.
ACS Material supplies exceptional advanced research materials to leading laboratories around the world. Our products have earned a reputation for high quality and consistency, and our customer service team is valued for their exceptional assistance before, during, and after an order is placed. Contact us today to find out more about how our products can help you move your research forward.
FAQ
1. How to disperse the Single Layer Graphene Powder into Aqueous Systems?
The SLG product produced by ACS Material has no functional groups on the surface‚ and it can be dispersed in water or ethanol with a dispersion aid. These two dispersion aids have been proven to be useful:
1) Sodium dodecylbenzene sulfonate (SDBS)
2) Sodium dodecyl sulfate (SDS)
Add dispersant to water‚ and then add single layer graphene with the use of an ultrasonic mixer. The addition rate of single layer graphene should be carefully controlled until the desired concentration is achieved. Experiments are needed to determine the appropriate quantity of dispersant in your system‚ but in our experience with water‚ 10mg/ml dispersant is preferred. The sonication time should be 15+ minutes. If you notice some settling‚ assuming you have not attempted to over-concentrate the dispersion‚ the graphene can be redispersed with the aid of sonication. The maximum concentration of single layer graphene in water is about 0.1 mg/ml with SDBS as dispersant‚ or 100mg/l.
2. Are there other elements in Single Layer Graphene?
Single Layer Graphene contains a bit of sulfur. Sulfuric acid is necessary for intercalation during the initial treatment and sulfur cannot be completely removed in the final product.
Research Citations of ACS Material Products
- Randeniya, Lakshman K., et al. “Harnessing the Influence of Reactive Edges and Defects of Graphene Substrates for Achieving Complete Cycle of Room-Temperature Molecular Sensing.” Small, vol. 9, no. 23, Jan. 2013, pp. 3993–3999., doi:10.1002/smll.201300689.
- Zhang, Lv, et al. “A tough graphene nanosheet/Hydroxyapatite composite with improved in vitro biocompatibility.” Carbon, vol. 61, 2013, pp. 105–115., doi:10.1016/j.carbon.2013.04.074.
- Li, Yunyong, et al. “Simultaneous Formation of Ultrahigh Surface Area and Three-Dimensional Hierarchical Porous Graphene-Like Networks for Fast and Highly Stable Supercapacitors.” Advanced Materials, vol. 25, no. 17, 2013, pp. 2474–2480., doi:10.1002/adma.201205332.
- Xu, Peng, et al. “Synergy among binary (MWNT, SLG) nano-Carbons in polymer nano-Composites: a Raman study.” Nanotechnology, vol. 23, no. 31, 2012, p. 315706., doi:10.1088/0957-4484/23/31/315706.
- Loomis, James, and Balaji Panchapakesan. “Dimensional dependence of photomechanical response in carbon nanostructure composites: a case for carbon-Based mixed-Dimensional systems.” Nanotechnology, vol. 23, no. 21, Mar. 2012, p. 215501., doi:10.1088/0957-4484/23/21/215501.
- Ip, Alexander C.-F., et al. “Oxidation Level-Dependent Zwitterionic Liposome Adsorption and Rupture by Graphene-Based Materials and Light-Induced Content Release.” Small, vol. 9, no. 7, 2012, pp. 1030–1035., doi:10.1002/smll.201202710.
- Xu, Peng, et al. “Load transfer and mechanical properties of chemically reduced graphene reinforcements in polymer composites.” Nanotechnology, vol. 23, no. 50, 2012, p. 505713., doi:10.1088/0957-4484/23/50/505713.
- Loomis, James, et al. “Layer dependent mechanical responses of graphene composites to near-Infrared light.” Applied Physics Letters, vol. 100, no. 7, 2012, p. 073108., doi:10.1063/1.3685479.
- Loomis, James, and Balaji Panchapakesan. “Large photocurrents in single layer graphene thin films: effects of diffusion and drift.” Nanotechnology, vol. 23, no. 26, 2012, p. 265203., doi:10.1088/0957-4484/23/26/265203.
- Chen, Qi, et al. “Enhanced Hot-Carrier Luminescence in Multilayer Reduced Graphene Oxide Nanospheres.” Scientific Reports, vol. 3, no. 1, 2013, doi:10.1038/srep02315.
- Xu, Peng, et al. “Synergy among binary (MWNT, SLG) nano-Carbons in polymer nano-Composites: a Raman study.” Nanotechnology, vol. 23, no. 31, 2012, p. 315706., doi:10.1088/0957-4484/23/31/315706.
- Aziz, Saad, et al. “Nanoarchitectured LiMn2O4/Graphene/ZnO Composites as Electrodes for Lithium Ion Batteries.” Journal of Materials Science & Technology, vol. 30, no. 5, 2014, pp. 427–433., doi:10.1016/j.jmst.2014.03.007.
- Lewandowski, Andrzej, et al. “Capacity of graphene anode in ionic liquid electrolyte.” Journal of Solid State Electrochemistry, vol. 18, no. 10, 2014, pp. 2781–2788., doi:10.1007/s10008-014-2539-3.
- Zuccon, Sara, et al. “Functional palladium metal films for plasmonic devices: an experimental proof.” Journal of Optics, vol. 16, no. 5, 2014, p. 055001., doi:10.1088/2040-8978/16/5/055001.
- Tampieri, Francesco, et al. “A comparative electron paramagnetic resonance study of expanded graphites and graphene.” J. Mater. Chem. C, vol. 2, no. 38, 2014, pp. 8105–8112., doi:10.1039/c4tc01383b.
- Sharma, Rajni, et al. “ZnO anchored graphene hydrophobic nanocomposite-Based bulk heterojunction solar cells showing enhanced short-circuit current.” Journal of Materials Chemistry C, 1 Aug. 2014, pp. 8142–8151., doi:10.1039/C4TC01056F.
- Majidian, Maryam, et al. “Electrical conduction of photo-Patternable SU8–graphene composites.” Carbon, vol. 80, 2014, pp. 364–372., doi:10.1016/j.carbon.2014.08.075.
- Zhang, Tao, et al. “Gel-Derived Cation-π Stacking Films of Carbon Nanotube-Graphene Complexes as Oxygen Cathodes.” ChemSusChem, vol. 7, no. 10, 2014, pp. 2845–2852., doi:10.1002/cssc.201402567.
- Swiderska-Mocek, Agnieszka, and Ewelina Rudnicka. “Lithium–sulphur battery with activated carbon cloth-Sulphur cathode and ionic liquid as electrolyte.” Journal of Power Sources, vol. 273, 2015, pp. 162–167., doi:10.1016/j.jpowsour.2014.09.020.
- Terborg, Lydia, et al. “Porous polymer monoliths with incorporated single layer graphene.” Scientia Chromatographica, vol. 6, no. 1, 2014, pp. 27–33., doi:10.4322/sc.2014.017.
- Sims, Christopher M., et al. “CO tolerance of Pt and PtSn intermetallic electrocatalysts on synthetically modified reduced graphene oxide supports.” Dalton Transactions, vol. 44, no. 3, 2015, pp. 977–987., doi:10.1039/c4dt02544j.
- Amoli, Behnam Meschi, et al. “Highly electrically conductive adhesives using silver nanoparticle (Ag NP)-Decorated graphene: the effect of NPs sintering on the electrical conductivity improvement.” Journal of Materials Science: Materials in Electronics, vol. 26, no. 1, Apr. 2014, pp. 590–600., doi:10.1007/s10854-014-2440-y.
- Amoli, Behnam Meschi, et al. “SDS-Stabilized graphene nanosheets for highly electrically conductive adhesives.” Carbon, vol. 91, 2015, pp. 188–199., doi:10.1016/j.carbon.2015.04.039.
- Wehnert, F., et al. “Design of multifunctional adhesives by the use of carbon nanoparticles.” Journal of Adhesion Science and Technology, vol. 29, no. 17, 2015, pp. 1849–1859., doi:10.1080/01694243.2015.1014536.
- Liang, Xiao, et al. “A highly efficient polysulfide mediator for lithium–sulfur batteries.” Nature Communications, vol. 6, June 2015, p. 5682., doi:10.1038/ncomms6682.
- Paczosa-Bator, Beata. “Ion-Selective electrodes with superhydrophobic polymer/Carbon nanocomposites as solid contact.” Carbon, vol. 95, 2015, pp. 879–887., doi:10.1016/j.carbon.2015.09.006.
- Bhatnagar, Deepika, et al. “Graphene quantum dots FRET based sensor for early detection of heart attack in human.” Biosensors and Bioelectronics, vol. 79, 2016, pp. 495–499., doi:10.1016/j.bios.2015.12.083.
- Saha, Dipendu, et al. “Adsorption of CO2, CH4, and N2 in Micro-Mesoporous Nanographene: A Comparative Study.” Journal of Chemical & Engineering Data, vol. 60, no. 9, Nov. 2015, pp. 2636–2645., doi:10.1021/acs.jced.5b00291.
- Saha, Dipendu, et al. “A study on the cytotoxicity of carbon-based materials.” Materials Science and Engineering: C , vol. 68, Nov. 2016, pp. 101–108., doi:10.1016/j.msec.2016.05.094.
- Alhumade, H., et al. “Enhanced protective properties and UV stability of epoxy/Graphene nanocomposite coating on stainless steel.” Express Polymer Letters, vol. 10, no. 12, 2016, pp. 1034–1046., doi:10.3144/expresspolymlett.2016.96.
- Deng, Wenfang, et al. “Three-Dimensional graphene-like carbon frameworks as a new electrode material for electrochemical determination of small biomolecules.” Biosensors and Bioelectronics, vol. 85, 2016, pp. 618–624., doi:10.1016/j.bios.2016.05.065.
- Alhumade, Hesham, et al. “Corrosion inhibition of copper in sodium chloride solution using polyetherimide/Graphene composites.” The Canadian Journal of Chemical Engineering, vol. 94, no. 5, 2016, pp. 896–904., doi:10.1002/cjce.22439.
- Pięk, Magdalena, et al. “The Complex Crystal of NaTCNQ–TCNQ Supported on Different Carbon Materials as Ion-to-Electron Transducer in All-Solid-State Sodium-Selective Electrode.” Journal of The Electrochemical Society, vol. 163, no. 13, 2016, doi:10.1149/2.0341613jes.
- Albelda, Jasmine A.V, et al. “Graphene-Titanium dioxide nanocomposite based hypoxanthine sensor for assessment of meat freshness.” Biosensors and Bioelectronics, vol. 89, 2017, pp. 518–524., doi:10.1016/j.bios.2016.03.041.
- Dubey, Nileshkumar, et al. “Graphene: An Emerging Carbon Nanomaterial for Bone Tissue Engineering.” Graphene-Based Materials in Health and Environment Carbon Nanostructures, 2016, pp. 135–158., doi:10.1007/978-3-319-45639-3_5.
- Dobrota, Ana S., et al. “Stabilization of alkali metal ions interaction with OH-Functionalized graphene via clustering of OH groups – implications in charge storage applications.” RSC Advances, vol. 6, no. 63, 2016, pp. 57910–57919., doi:10.1039/c6ra13509a.
- Mohan, Balaji, et al. “Mechanochemical Synthesis of Active Magnetite Nanoparticles Supported on Charcoal for Facile Synthesis of Alkynyl Selenides by C−H Activation.” ChemCatChem, vol. 8, no. 14, 2016, pp. 2345–2350., doi:10.1002/cctc.201600280.
- Pięk, Magdalena, et al. “All-Solid-State nitrate selective electrode with graphene/Tetrathiafulvalene nanocomposite as high redox and double layer capacitance solid contact.” Electrochimica Acta, vol. 210, 2016, pp. 407–414., doi:10.1016/j.electacta.2016.05.170.
- Marinoiu, A., et al. “Iodinated carbon materials for oxygen reduction reaction in proton exchange membrane fuel cell. Scalable synthesis and electrochemical performances.” Arabian Journal of Chemistry, 2016, doi:10.1016/j.arabjc.2016.12.002.
- Pu, Zhihua, et al. “A continuous glucose monitoring device by graphene modified electrochemical sensor in microfluidic system.” Biomicrofluidics, vol. 10, no. 1, 2016, p. 011910., doi:10.1063/1.4942437.
- Gatti, Teresa, et al. “Boosting Perovskite Solar Cells Performance and Stability through Doping a Poly-3(Hexylthiophene) Hole Transporting Material with Organic Functionalized Carbon Nanostructures.” Advanced Functional Materials, vol. 26, no. 41, 2016, pp. 7443–7453., doi:10.1002/adfm.201602803.
- Nieto, Andy, et al. “Graphene reinforced metal and ceramic matrix composites: a review.” International Materials Reviews, vol. 62, no. 5, 2016, pp. 241–302., doi:10.1080/09506608.2016.1219481.
- Marinoiu, Adriana, et al. “Doped Graphene as Non-Metallic Catalyst for Fuel Cells.” Materials Science, vol. 23, no. 2, 2017, doi:10.5755/j01.ms.23.2.16216.
- Kang, Shin Wook, et al. “High-Performance Fe 5 C 2 @CMK-3 nanocatalyst for selective and high-Yield production of gasoline-Range hydrocarbons.” Journal of Catalysis, vol. 349, 2017, pp. 66–74., doi:10.1016/j.jcat.2017.03.004.
- Pięk, Magdalena, et al. “High selective potentiometric sensor for determination of nanomolar con-Centration of Cu(II) using a polymeric electrode modified by a graphene/7,7,8,8-Tetracyanoquinodimethane nanoparticles.” Talanta, vol. 170, 2017, pp. 41–48., doi:10.1016/j.talanta.2017.03.068.
- Marinoiu, Adriana, et al. “Low cost iodine intercalated graphene for fuel cells electrodes.” Applied Surface Science, vol. 424, 2017, pp. 93–100., doi:10.1016/j.apsusc.2017.01.295.
- Liu, Yangshuai, et al. “Asymmetric supercapacitor, based on composite MnO 2 -Graphene and N-Doped activated carbon coated carbon nanotube electrodes.” Electrochimica Acta, vol. 233, 2017, pp. 142–150., doi:10.1016/j.electacta.2017.03.028.
- Huang, Kun, et al. “Graphene coupled with Pt cubic nanoparticles for high performance, air-Stable graphene-Silicon solar cells.” Nano Energy, vol. 32, 2017, pp. 225–231., doi:10.1016/j.nanoen.2016.12.042.
- Deng, Wenfang, et al. “Three-Dimensional nitrogen-Doped graphene derived from poly-o-Phenylenediamine for high-Performance supercapacitors.” Journal of Electroanalytical Chemistry, vol. 787, 2017, pp. 103–109., doi:10.1016/j.jelechem.2017.01.047.
- Müller, Michael, et al. “Effect of Graphite Nanoplate Morphology on the Dispersion and Physical Properties of Polycarbonate Based Composites.” Materials, vol. 10, no. 6, 2017, p. 545., doi:10.3390/ma10050545.
- Chakrabarti, Barun, et al. “Enhanced Performance of an All-Vanadium Redox Flow Battery Employing Graphene Modified Carbon Paper Electrodes.” International Journal of Chemical and Molecular Engineering, vol. 11, no. 9, 2017, doi:10.1999/1307-6892/10007796.
- Gatti, Teresa, et al. “A D-π-A organic dye – Reduced graphene oxide covalent dyad as a new concept photosensitizer for light harvesting applications.” Carbon, vol. 115, 2017, pp. 746–753., doi:10.1016/j.carbon.2017.01.081.
- Zhou, Yikang, et al. “Graphene-Doped polyaniline nanocomposites as electromagnetic wave absorbing materials.” Journal of Materials Science: Materials in Electronics, vol. 28, no. 15, May 2017, pp. 10921–10928., doi:10.1007/s10854-017-6872-z.
- Das, Suprem R., et al. “Electrical Differentiation of Mesenchymal Stem Cells into Schwann-Cell-Like Phenotypes Using Inkjet-Printed Graphene Circuits.” Advanced Healthcare Materials, vol. 6, no. 7, 20 Feb. 2017, doi:10.1002/adhm.201601087.
- Kafiah, Feras, et al. “Synthesis of Graphene Based Membranes: Effect of Substrate Surface Properties on Monolayer Graphene Transfer.” Materials, vol. 10, no. 1, 2017, p. 86., doi:10.3390/ma10010086.
- Marinoiu, Adriana, et al. “Doped Graphene as Non-Metallic Catalyst for Fuel Cells.” Materials Science, vol. 23, no. 2, 2017, doi:10.5755/j01.ms.23.2.16216.
- Casalino, M., et al. “Free-Space graphene/Silicon photodetectors operating at 2 micron.” 15 Nov. 2017.
- Pu, Zhihua, et al. “A Wearable Continuous Glucose Monitoring System with Electrochemical Sensor Modified by Graphene.” Oct. 2015.
- Alhumade, Hesham, et al. “Proceedings of the 2016 International Conference on Industrial Engineering and Operations Management.” Optimizing Corrosion Protection of Stainless Steel 304 by Epoxy-Graphene Composite using Factorial Experimental Design.
- Slamet, and Raudina. “Degradation of 2,4,6-Trichlorophenol and hydrogen production simultaneously by TiO2 nanotubes/Graphene composite.” 2017, doi:10.1063/1.5011931.
- Marinoiu, Adriana, et al. “Low cost iodine intercalated graphene for fuel cells electrodes.” Applied Surface Science, North-Holland, 31 Jan. 2017, www.sciencedirect.com/science/article/pii/S0169433217303227.
- Ha, Trung B., et al. “Electro-Immobilization of Acetylcholinesterase Using Polydopamine for Carbaryl Microsensor.” Journal of Electronic Materials, vol. 47, no. 2, 2017, pp. 1686–1693., doi:10.1007/s11664-017-5880-3.
- Guarracino, Paola, et al. “Probing photoinduced electron-Transfer in graphene–dye hybrid materials for DSSC.” Phys. Chem. Chem. Phys., vol. 19, no. 40, 2017, pp. 27716–27724., doi:10.1039/c7cp04308b.
- Lu, Bingyu, et al. “Side-Polished fiber SPR sensor with tempetrature self-Compensation for continuous glucose monitoring.” 2016 IEEE 29th International Conference on Micro Electro Mechanical Systems (MEMS), 2016, doi:10.1109/memsys.2016.7421648.
- Huang, Kun, et al. “High and Fast Response of a Graphene-Silicon Photodetector Coupled with 2D Fractal Platinum Nanoparticles.” Advanced Optical Materials, vol. 6, no. 1, Jan. 2017, p. 1700793., doi:10.1002/adom.201700793.
- Hondred, John A., et al. “High-Resolution Graphene Films for Electrochemical Sensing via Inkjet Maskless Lithography.” ACS Nano, vol. 11, no. 10, 2017, pp. 9836–9845., doi:10.1021/acsnano.7b03554.
- Seehra, Mohindar S., et al. “Correlation between X-Ray diffraction and Raman spectra of 16 commercial graphene–based materials and their resulting classification.” Carbon, vol. 111, 2017, pp. 380–385., doi:10.1016/j.carbon.2016.10.010.
- Yadav, Shriniwas, and Inderpreet Kaur. “Low temperature processed graphene thin film transparent electrodes for supercapacitor applications.” RSC Advances, vol. 6, no. 82, 2016, pp. 78702–78713., doi:10.1039/c6ra17668b.
- He, Qing, et al. “Enabling Inkjet Printed Graphene for Ion Selective Electrodes with Postprint Thermal Annealing.” ACS Applied Materials & Interfaces, vol. 9, no. 14, 2017, pp. 12719–12727., doi:10.1021/acsami.7b00092.
- Barbon, Antonio, and Francesco Tampieri. “Identification of slow relaxing spin components by pulse EPR techniques in graphene-Related materials.” AIMS Materials Science, vol. 4, no. 1, 2017, pp. 147–157., doi:10.3934/matersci.2017.1.147.
- Ali, Nafisa, Priyabrata Pal, Fawzi Banat, and Chandrasekar Srinivasakannan. "Selective removal of diethanolamine from methyldiethanolamine solution using chemically reduced single-layer graphene and activated carbon." Separation Science and Technology (2018): 1-11.
- Casalino, Maurizio, Roberto Russo, Carmela Russo, Anna Ciajolo, Emiliano Di Gennaro, Mario Iodice, and Giuseppe Coppola. "Free-Space Schottky Graphene/Silicon Photodetectors Operating at 2 μm." ACS Photonics 5, no. 11 (2018): 4577-4585.
- Paustovsky, A. V., V. E. Sheludko, E. Ya Telnikov, A. K. Marchuk, V. V. Kremenitsky, O. P. Tarasyuk, and S. P. Rogalsky. "The Structure and Electrical Properties of Graphene-Containing Thick Films." Powder Metallurgy and Metal Ceramics 57, no. 5-6 (2018): 285-292.
- Hoeprich Jr, Paul D., and Sangil Kim. "Electrochemical flow-cell for hydrogen production and nicotinamide dependent target reduction, and related methods and systems." U.S. Patent Application 14/861,750, filed March 24, 2016.
- Kinastowska, K., K. Piela, M. Gordel, A. Żak, R. Kołkowski, and M. Samoć. "Gold nanoparticle-decorated graphene as a nonlinear optical material in the visible and near-infrared spectral range." Physical Chemistry Chemical Physics 20, no. 27 (2018): 18862-18872.
